

EQUILIN, from electrodes to non-contact measurement. How to develop a disruptive medical device in 16 months
Autores: Adrián Morales Casas, Amparo López Vicente, Úrsula Martínez Iranzo, Daniel Gómez López, Juan Manuel Belda Lois, David Rubio, David Moro Pérez, Salvador Pitarch Corresa, Alfredo Gao Yao, Carlos Chirivella Moreno, José David Garrido Jaén, Carlos Atienza Vicente, Alejandro Gamón Sanz, José Laparra Hernández
Instituto de Biomecánica (IBV)
For more than 40 years, the IBV has applied human-centred methodologies that combine knowledge of Human Factors (HF) and User Experience (UX) to maximise the extent to which products, services and environments meet users’ needs.
This article explains how we adapted and optimised these methodologies to achieve the synergies needed to develop an innovative non-contact vital signs monitoring system, taking into account the regulatory requirements of the stringent European Medical Device Regulation (EU) 2017/745. The aim is to highlight the use of new medical device design methodologies, specifically aimed at solving the problems and cost overruns faced by developers, particularly when it comes to incorporating regulatory and usability aspects into their designs.
It is precisely the lack of fit between the product and the users that often has critical consequences, increasing risks and escalating development costs. This is why the Instituto de Biomecánica (IBV) advocates the use of methodologies such as Lean, Agile, User Experience (UX) and Human Factors (HF), which have been shown to optimise development processes, reduce costs and generate value.
The success story we wish to present is the EQUILIN project, developed jointly with the 061 Emergency Care Centre of the Andalusian Health Service, in which these methods were applied in three phases, resulting in a functional prototype of a device that was then subjected to real-life validation over a period of 16 months, for use in out-of-hospital emergencies.
Weighing less than 500 grams and with an 8-hour battery life and an intuitive interface, the system provides non-contact measurement of 5 key vital signs in a variety of emergency care environments.
INTRODUCTION
More than 88% of medical device developers fail to generate a positive return on investment after market launch (Marešová et al., 2020), partly because their activities are focused on regulatory compliance without an optimised process (World Health Organization, 2010), and partly because of a lack of fit between the functionalities and usability of the products and their users. This is a critical factor in the case of medical devices due to the potential risk that they may cause adverse effects in humans, including disability or even death (Roma and de Vilhena Garcia, 2020), a risk that increases exponentially as the products become more complex and incorporate new technologies.
In this context, it is important to highlight that obtaining the CE marking for a medical device, which is a prerequisite for its marketing in Europe, can entail a development effort of between 2 and 3 years for companies, which means that delays due to product design errors can prevent them from reaching the market on time, exponentially increasing the sunk costs of their development.
Despite this fact, most of these companies’ development strategies focus on meeting planned technical milestones in order to move towards a final solution, without taking into account the stakeholders involved in its use, be they patients, prescribers or healthcare professionals. Nevertheless, approaches or methodologies such as Agile Software Development, Lean Principles, User Experience (UX) and Human Factors (HF) allow us to generate more value and reduce costs.
Given this reality, IBV takes an approach to product development that combines these methodologies with its vision of human-centred design and innovation, with the aim of optimising the balance between quality, cost and development time (Figure 1).
This way of working has produced very relevant success stories in different sectors, from the Biomechanics[1] range of children’s shoes to the disruptive multifunctional station for Metro de Madrid[2], not to mention the health sector, especially since the approval of the demanding European Medical Device Regulation (EU) 2017/745[3] governing the launch of new products
Figure 1. Benefits in the development and market introduction of any medical device.
One of the most important projects of this type that the IBV has undertaken is EQUILIN, carried out in collaboration with the 061 Emergency Care Centre of the Andalusian Health Service, which posed a major scientific and technological challenge: The contact-based vital signs systems used in emergency departments today are heavy and bulky. Is it possible to replace them with a non-contact solution that is easy to use and suitable for use in a variety of environments? The outcome of the project was the development of a real-world validated prototype (TRL7) capable of measuring five key vital parameters (heart rate, respiratory rate, body temperature, oxygen saturation and blood pressure) without any contact with the patient and within 16 months.
This challenge was implemented through a Public Procurement of Innovative solutions, , a temporary joint venture led by the IBV, where a high-performance multidisciplinary team was assembled to apply the UX and FH methodologies, combined with the techniques of the Lean and Agile approaches.
The development project was split into 3 phases: understanding the problem, generating ideas, and designing and validating the final solution through an iterative process:
- Phase 1. Learn, to understand the work and needs of the professionals and their context.
- Phase 2. Ideate, to generate new human-centred solutions.
- Phase 3. Validate, in all three phases, of the adaptation of the medical device to the needs of patients and professionals.
METHODOLOGY
The methodology used during the project, which led to the successful development of a non-contact monitoring system for the measurement of vital parameters (Figure 2), is based on the application of the three previous phases, which are described below.

Figure 2. Final version of the prototype of the non-contact monitoring system resulting from the EQUILIN project.
Phase 1. Learn: Understanding the need for and the context in which the registration system is used
This phase focused on understanding the day-to-day work and needs of the staff that make up the 061 Emergency Care Centre of the Andalusian Health Service, including professionals from different areas, from healthcare to logistics and management, as well as patients. During this phase, an in-depth analysis of how the out-of-hospital emergency service is provided was carried out. The analysis involved stakeholders, and the aim was to obtain a sufficient number of case histories in each of the defined scenarios: ambulances, patient’s homes, outdoor emergencies and accidents involving multiple casualties.
This analysis included field studies and observations by IBV staff, and the use of techniques such as focus groups, individual interviews and meetings with experts in the sector. Figure 3 shows real situations collected during the observation sessions, which made it possible to identify the possible difficulties that healthcare professionals may face and potential improvements to the equipment currently used in the service to monitor vital signs, highlighting the drawbacks related to volume and weight, as well as the length of time required to instrument patients and assess their risk using contact monitoring systems, a critical factor in assessing the condition of patients in an emergency.
Figure 3. Examples of situations faced on a daily basis by healthcare professionals at the 061 Emergency Care Centre of the Andalusian Health Service.
Phase 2. Ideate: from the needs to the definition of a solution
Phase two consisted of setting out the initial ideas for the measurement system and developing them to the point where the first functional prototypes of the solution could be defined, based on the requirements generated in the previous phase.
This phase required several iterations and the participation of patients and HF and UX experts from the IBV, professionals involved in their daily use. To this end, the IBV organised several co-creation sessions with healthcare professionals, which produced ideas that were subsequently evaluated by other healthcare professionals and led to the creation of Minimum Viable Products (MVPs) that were validated by the main stakeholders involved in the project.
The aim of these sessions was to incrementally develop a system that would meet the needs of the professionals in terms of functionality and usability, while meeting the technical requirements defined in the project. Figure 4 shows examples of a few of the initial ideas that were developed during one of the co-creation sessions, and figure 5 shows how these initial ideas evolved into the first functional prototypes that led to the final solution.
Figure 4. Examples of some of the ideas generated by the co-creation sessions using the Lego® Serious Play technique.
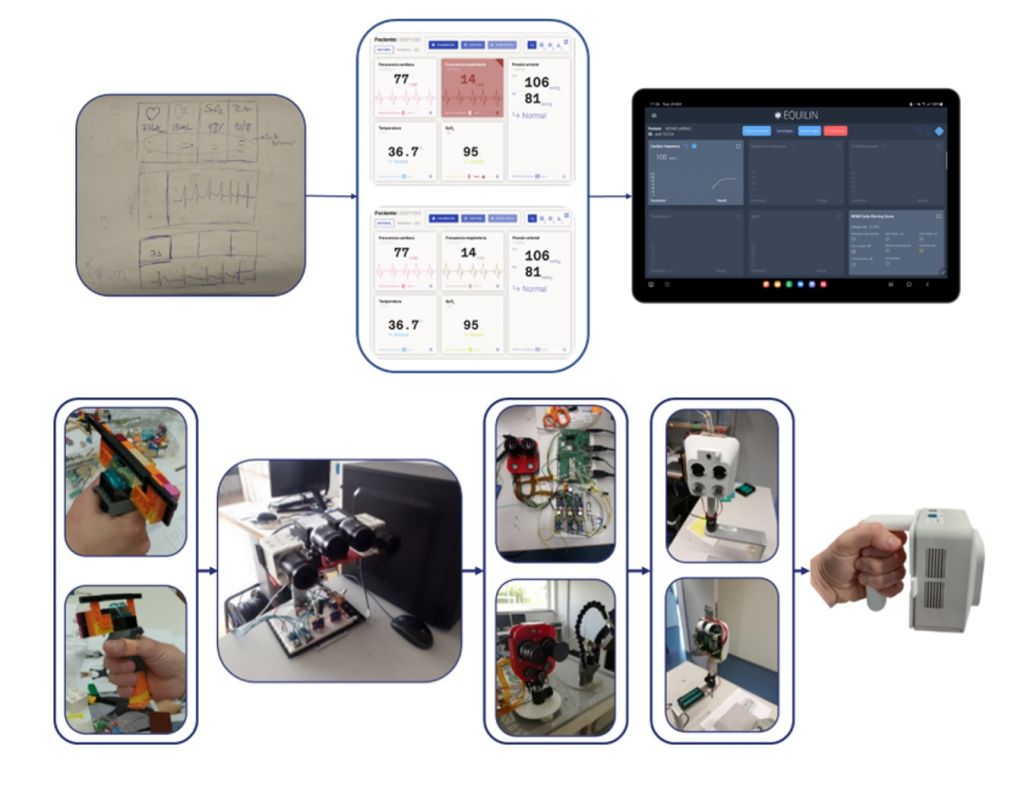
Figure 5. Evolution of the solution from the initial ideas to the final prototype, both in terms of software (top image) and hardware (bottom image).
In this phase, the system that had been developed was first tested in the laboratory on a sample of 70 users, distributed according to gender, age, skin colour and body mass index, and in different usage scenarios. Not only did this experiment validate the results of the system, but it also allowed the IBV to create the world’s only structured database of non-contact measurements (the Freyja Dataset, owned by the IBV).
Phase 3. Validation: from the laboratoy to the real world
Iterative validation activities began in the previous phase: from the evaluation of the first conceptual sketches, to tests on non-functional prototypes ( in order to evaluate aspects such as the weight of the system, its dimensions or the layout of the indicators on the screen), to laboratory tests (in order to evaluate the quality of the signals monitored and the information displayed or to identify possible artefacts resulting from the movement of the professional using it). However, it is at this stage that the in-situ testing of the functional versions of the system (under controlled conditions and the supervision of IBV staff) and the final validation tests in a real-world environment by healthcare professionals are considered.
These validations made it possible to confirm design choices, detect errors at an early stage and increase user satisfaction, thereby increasing the likelihood of acceptance by all stakeholders and the success of the product on the market. For example, the results of the in-situ validation of the first functional prototype were highly satisfactory, with the majority of evaluations close to or above 90% in terms of the system’s compliance with its intended functions, its usefulness, comfort and ease of use, and its improvement on the problems identified in devices currently on the market.
The final stage of this phase was the validation of the system under real conditions on more than 300 patients between May and July 2023. This validation demonstrated the quality of the results obtained in the monitoring of non-contact signals and the robustness of the system when used in the demanding out-of-hospital emergency care process. The validation took place in different scenarios: at the patient’s home, outdoors, during the ambulance journey to the health centre and during the management of multiple casualty situations. These tests made it possible to identify minor improvements, which were implemented before the functional prototype was deemed complete.
RESULTS
By applying the IBV methodology, it was possible to develop from scratch, in just 16 months, and validate in the real and particularly complex environment of an out-of-hospital emergency care unit, an innovative non-contact vital signs monitoring system that is unique in the market. Thanks to its small size and manoeuvrability, the device can be used by healthcare professionals, installed in ambulances or even mounted on a robotic dog in a specific emergency.
The device developed weighs less than 500 grams, has an 8-hour battery life and makes it possible to monitor physiological variables, either episodically or continuously, depending on the precision requirements defined at the start of the project. It also has an intuitive and easy-to-use interface that displays the patient’s status and manages multimodal alerts (colour, light and sound changes) in the event of a risk situation due to critical changes in the patient’s recorded signals in real time.
In terms of the reliability of the system’s results, very high levels of accuracy – between 75 and 100% – have been achieved for all the variables recorded. The best results are for heart rate, respiratory rate and body temperature. Work is ongoing to improve the reliability of the blood pressure and oxygen saturation results through complementary studies for use in different pathologies.
It is important to note that the suggested improvements and usage issues identified during the three development phases allow for ongoing changes to be made at a lower cost and with no impact on the schedule, facilitating a design that is focused on the needs of healthcare professionals to ensure a good reception in the market. These results, together with the level of satisfaction achieved during the validation phase, represent a major advantage in the development of disruptive products.
In summary, the main results of the development of the system are as follows:
- Improved efficiency of out-of-hospital emergency services: the system reduces patient assessment times from 5 minutes to 30 seconds.
- Reduced risk to healthcare professionals: as no contact is required, the risk of healthcare professionals contracting infectious diseases is minimised. On the other hand, the reduction in weight compared to current systems (from an average of 4 kilograms for commercial devices to 500 grams for the system developed in the project) avoids ergonomic risks.
- Improved robustness and reliability of measurements: the precision of the results obtained by the developed system is within the uncertainty range of products currently on the market.
- User acceptance: high acceptance by professionals and patients in the different usage scenarios. Many of them agree that “the result of the EQUILIN project must be incorporated into the healthcare of the future“.
CONCLUSIONS
IBV’s experience in using a combination of UX, HF, Lean and Agile methodologies made it possible to achieve an extremely complex objective: to develop a system capable of monitoring vital signs without contact in 16 months, identifying improvements and problems throughout the development process in order to achieve 0% errors in use and 100% user satisfaction.
ACKNOWLEDGEMENTS
We would like to dedicate the results of this project to all the healthcare professionals at the 061 Emergency Care Service of the Andalusian Health Service for their active involvement in the project.
‘The EQUILIN project is promoted by the 061 Emergency Care Centre of the Andalusian Health Service, with a total budget of 1 million euros, 80% co-financed by the European Regional Development Fund (ERDF) through the Spanish Multi-regional Operational Programme (POPE) 2014-2020, aid granted by the Ministry of Science and Innovation through the FID programme ‘Promoting Innovation from the Demand Side’.
BIBLIOGRAPHY
[1] https://www.biomecanicamente.org/news/ibv/item/1476-biomecanics-el-calzado-para-aprender-a-andar-que-triunfa-en-espana.html
[2] https://www.europapress.es/madrid/noticia-metro-madrid-reconocido-internacionalmente-nuevos-sistemas-acceso-estaciones-20220703111452.html
[3] La MDR es Reglamento (UE) 2017/745 del Parlamento Europeo y del Consejo, de 5 de abril de 2017, sobre los productos sanitarios, por el que se modifican la Directiva 2001/83/CE, el Reglamento (CE) n.° 178/2002 y el Reglamento (CE) n.° 1223/2009 y por el que se derogan las Directivas 90/385/CEE y 93/42/CEE del Consejo
Marešová, P., Klímová, B., Honegr, J., Kuča, K., Ibrahim, W. N. H., & Selamat, A. (2020). Medical Device Development Process, and Associated Risks and Legislative Aspects-Systematic Review. Frontiers in Public Health, 8, 308.
Roma, M.S.G., de Vilhena Garcia, E. Medical device usability: literature review, current status, and challenges. Res. Biomed. Eng. 36, 163–170 (2020).
World Health Organization. (2010). Medical devices: managing the mismatch: an outcome of the Priority Medical Devices project. World Health Organization.
AFFILIATION OF AUTHORS
Instituto de Biomecánica de Valencia
Universitat Politècnica de València
Edificio 9C. Camino de Vera s/n
(46022) Valencia. Spain








