

PROSTACTO: Development of a prostate cancer screening device
17 February 2025.
Autor/es: Víctor J. Primo Capella, Adrián Morales Casas, Carlos M. Atienza Vicente, José Luis Peris Serra, Juan López Pascual.
Instituto de Biomecánica (IBV)
Cancer is the main cause of morbidity and mortality in Spain, and prostate cancer is the most common in men, with more than 30,000 cases diagnosed each year. Although early detection has improved significantly, traditional methods, such as a rectal examination, have inherent limitations due to their subjectivity and lack of precision, highlighting the need for more objective and accessible tools. The PROSTACTO initiative addresses this challenge. This project involves the development of an innovative prostate cancer screening device based on an objective assessment of prostate tissue stiffness using state-of-the-art sensors and ultrasound, in a portable and economical format.
The participants in the initiative are the Instituto de Biomecánica (IBV), the La Fe Health Research Institute (IIS La Fe), the Polytechnic University of Valencia (UPV), the Institute of Instrumentation for Molecular Imaging (I3M), the Spanish National Research Council (CSIC) and the venture builder REDIT Ventures, with the financial support of the Valencian Agency for Innovation (AVI), now known as IVACE+i. The outcome of this joint effort will be the commercialisation of the device by the start-up company RI MEDICAL.
INTRODUCTION
Cancer remains one of the main causes of morbidity and mortality in the world and in Spain. In 2020, approximately 18.1 million new cases of cancer were diagnosed worldwide (excluding non-melanoma skin tumours) and the number of new diagnoses is expected to increase to 28 million new cases per year by 2040[1].
The annual report of the Spanish Society of Medical Oncology estimated that the number of cases in Spain would rise to 286,664 in 2024, a slight increase over 2023. This increase is attributed to several factors such as population growth, ageing, exposure to risk factors (tobacco, alcohol, obesity and a sedentary lifestyle) and, in certain cancers such as colorectal, breast, cervical or prostate cancer, an increase in early detection[2].
The same report states that the most frequently diagnosed cancers in Spain in 2024 were colorectal cancer (44,294 new cases), breast cancer (36,395), lung cancer (32,768), prostate cancer (30,316) and urinary bladder cancer (22,097). In men, the most frequently diagnosed cancer was prostate cancer, followed by colorectal cancer, lung cancer and urinary bladder cancer[3].
A similar trend can be seen in the Valencian Community. Prostate cancer is the third most common cancer in the world, and, together with colorectal cancer, the most common cancer in men, accounting for 30% of all tumours in men[4].
In the Valencian Community, figures recorded since 2007 show a decrease in the incidence rate, which is now 130.3 cases per 100,000 inhabitants, lower than the European and Spanish rates. These figures mean that 1 in 14 men in the Valencian Community may develop prostate cancer[5].
Rectal examination as a screening methog
As mentioned above, the increase in the number of patients diagnosed with cancer is related to the increase in early detection, thanks to the existing detection programmes, as in the case of breast or prostate cancer. In the latter case, one aspect of the screening process that remains unresolved is the rectal examination. Rectal examination was the key component of prostate cancer detection programmes before blood tests to detect prostate specific antigen (PSA) became the generally accepted method. However, this technique has a drawback in that it is unpleasant for both the examiner and the patient.
Another problem inherent to rectal examinations is their subjectivity. Therefore, the existence of a complementary technique capable of objectifying the result of a rectal examination would be a considerable step forward in the detection of prostate cancer in the context of normal PSA and in the presence of localised indurations in difficult-to-examine regions.
In this context, and taking this clinical need as its starting point, the PROSTACTO initiative is the result of a collaboration between the La Fe Health Research Institute (IIS La Fe), the Instituto de Biomecánica (IBV), the Institute of Instrumentation for Molecular Imaging (I3M) and the Polytechnic University of Valencia (UPV). This initiative involves the development of a device that makes it possible to objectify a rectal examination as a simple and rapid method of screening for prostate cancer in the primary care context.
This article presents the development and the main milestones achieved in recent years as a result of this collaboration, which have culminated in the transfer of a patent to the newly created company, RI Medical, for its commercial exploitation.
DEVELOPMENT AND EVALUATION OF THE DEVICE
First steps
The design and development of the PROSTACTO device began as part of a collaborative project between REDIT and the Urology Department of the La Fe Hospital. In this initial project, the consortium consisted of a clinical team attached to the La Fe Hospital and the IBV. The proposal envisaged the development of a device designed to facilitate the screening of patients with prostate cancer. This device had to meet three initial requirements: it had to be portable, economical and easy to use by medical staff who were not specialists in urology. These characteristics would make it easier to add the device to the resources available in primary care for the early screening or detection of prostate cancer.
Based on these premises, the IBV set out to design a device that would measure stiffness by indenting the prostate tissue, with the aim of detecting possible alterations in the stiffness of such tissue caused by the presence of cancerous tissue.
Following several design iterations in collaboration with the clinical team, the design was split into two main parts. On the one hand, a handle with which to hold the device during the examination, designed to house all the electronics required for its use. On the other hand, the main cylinder attached to the handle, designed to house the exploration probe used to assess stiffness and the associated sensors (Figure 1).
The exploration probe is designed to measure the force exerted on two concentric sensors: the ratio between the forces registered by the two sensors is the value used to determine whether a tissue is more or less stiff. The greater the ratio between the two forces, the greater the stiffness of the system and therefore the greater the risk that the tissue is a tumour.
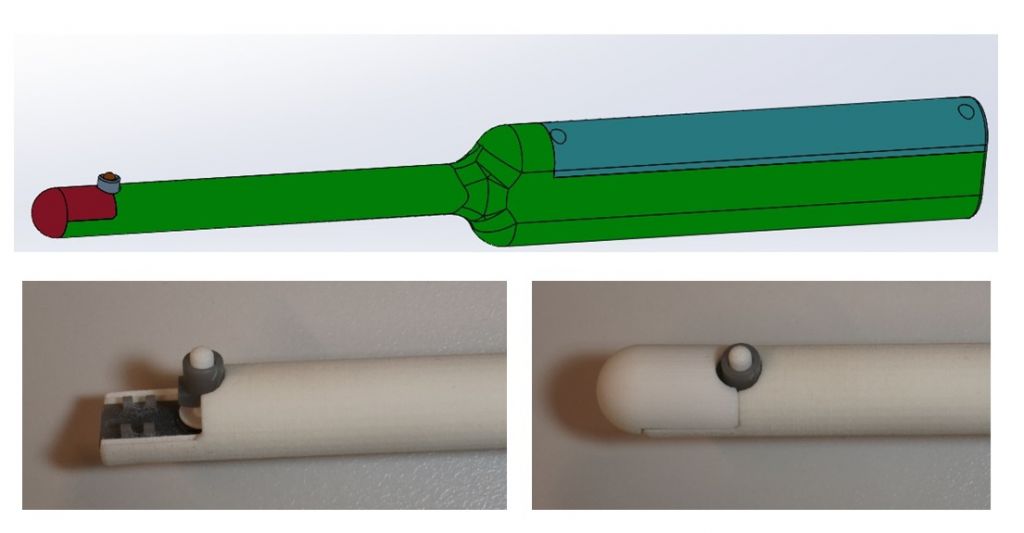
Figure 1. Initial design of the PROSTACTO device. Close-up of the exploration probe.
As the prototypes were being developed, the functionality of the device was tested using silicone phantoms in an effort to reproduce both the mechanical characteristics of healthy prostate tissue and those of pathological tissue, by incorporating materials of high stiffness and different sizes to simulate the indurations typical of such tissue.
In addition to the silicone phantoms, commercial phantoms designed for medical training in rectal examinations were also used (Figure 2).

Figure 2. Tests on silicone phantoms, commercial phantoms and explanted prostates.
cancer (Figure 2). Figure 3 shows two graphs of the measurements obtained from this explant in which the force recorded with the external sensor is plotted in blue, and the force recorded with the internal sensor is plotted in orange. The graph on the left shows a high ratio between the recorded forces, corresponding to an area of considerable stiffness which may indicate the presence of a tumour. The graph on the right shows a lower ratio, corresponding to tissue of normal stiffness.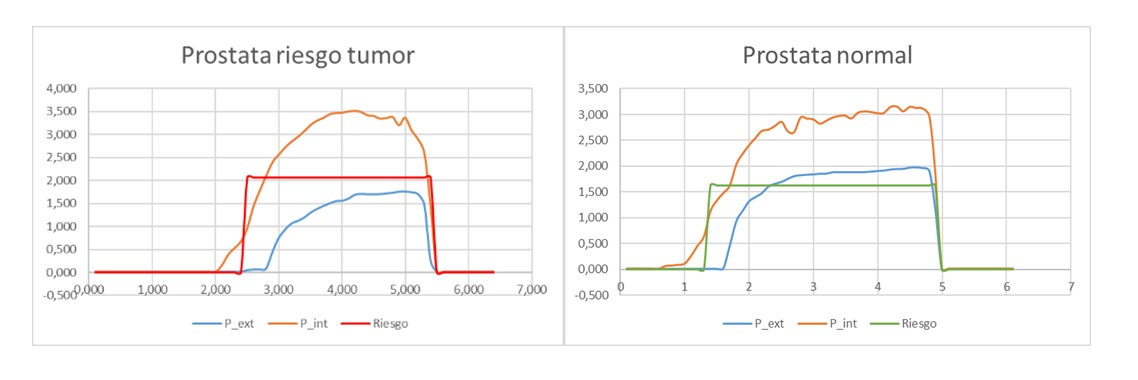
Figure 3. Examples of the recordings obtained from the device during the measurement of the explanted prostate.
Further development of the design
The design was further developed in a second project funded by the Valencian Innovation Agency (AVI), now known as IVACE+i, through a valorisation project. A third member joined the consortium of the previous project: the Institute of Instrumentation for Molecular Imaging (I3M/UPV).
The aim of this second project was to continue the development of the device, to introduce the improvements identified during the first phases of development and testing, and to carry out a clinical research study on patients in order to clinically validate its operation. An international patent covering the main target markets was also filed.
The design improvements focused on three aspects. The first was the incorporation of a retractable system so that the exploration probe could be folded in and out. This system was designed to protect the patient from potential injury during the examination, by allowing the probe to be deployed only during the actual measurement.
The second design improvement was the incorporation of an ultrasound probe. After initial tests on phantoms and explanted prostates, two issues were identified as critical to ensuring a correct procedure for analysing the prostate tissue: a) the orientation of the doctor during the examination and b) the location of the indentation zone. This design change had a direct impact on one of the main requirements that had been defined at the outset, namely that the device should be economical. Nevertheless, it was considered important and necessary enough to justify a change in the criteria. In order to carry out this modification, the original project consortium brought in the I3M research group, which specialises in the development of instrumentation and software algorithms for medical imaging.
The third and final major change was the decision to use biocompatible materials in the design and manufacture of the device. As mentioned above, the ultimate goal of the new project was to conduct a clinical research study to validate the performance of the device in a relevant clinical environment, which meant that the device to be tested had to meet minimum manufacturing requirements to ensure its safe use in patients.
In addition to these major changes, other design changes were made, particularly to the recording mechanism, such as changing the sensors used to record force from a double resistive ink sensor to a sensor based on the use of metal bands instrumented with strain gauges.
Figure 4 shows the final design of the device once the above changes had been implemented.
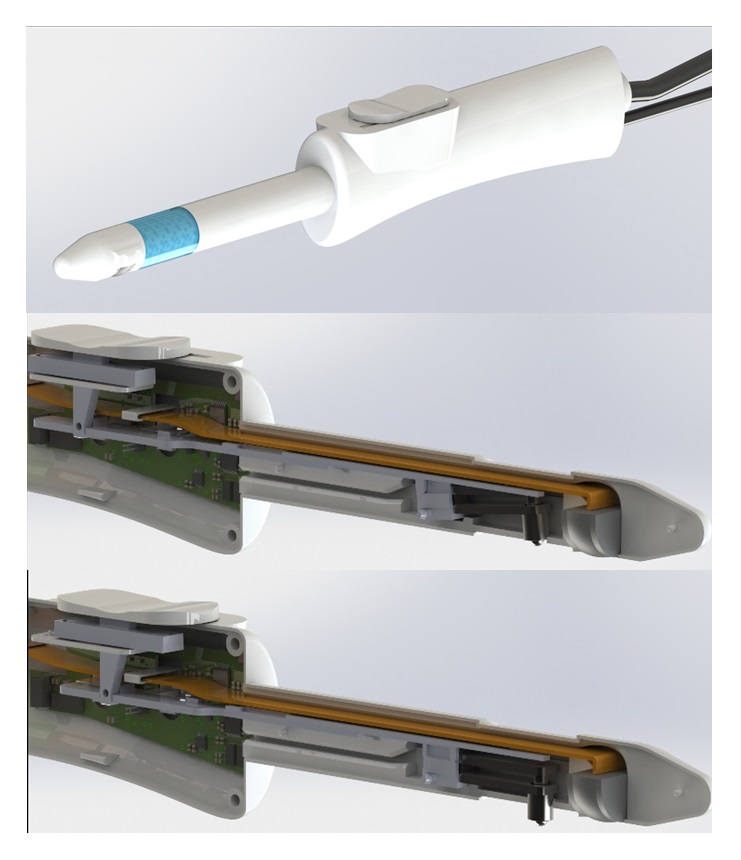
Figure 4. Final design of the PROSTACTO device used in the clinical research study.
As had been the case with the preliminary versions, once the functional prototype of the device had been designed and manufactured, an in vitro evaluation was carried out to verify its measurement functionality.
A clinical research study was then conducted on explanted prostates from patients diagnosed with prostate cancer and scheduled for a radical prostatectomy. The study was carried out in the facilities of the University and Polytechnic Hospital of La Fe by the staff of the Urology Department involved in the project, led by Dr César David Vera Donoso, and the research staff of the IBV.
This study involved the ex vivo examination of a total of 8 explanted prostates that had been previously examined by medical professionals and then examined with the device. This study allowed the device’s measurement parameters to be adjusted to better reflect the real characteristics of the tissue being examined.
Clinical validation of the device
Following the in vivo and ex vivo characterisation, all the technical documentation related to the design, manufacture and safety was prepared and compiled. This documentation was required to be able to apply for a review of the research study in patients by the ISS La Fe Medical Research Ethics Committee and the AEMPS.
Following approval by both organisations, and after two months of patient recruitment and measurements, the clinical research study was completed in early 2024. The study showed that exploring prostate tissue with the study device did not cause any harm to the patient and was therefore a safe exploration method.
During the explorations with the PROSTACTO device, potential improvements were identified, mainly related to the integration of the ultrasound probe into the measurement device and its placement in relation to the exploration probe. These and other improvements were addressed in subsequent redesign phases.
CONCLUSIONS
Following the completion of the two collaborative projects financed by regional funds, the Institute of Biomechanics (IBV), the La Fe Health Research Institute (IIS La Fe), the Polytechnic University of Valencia (UPV) and the Spanish National Research Council (CSIC), together with the venture builder REDIT Ventures, have created a new technology company, RI MEDICAL, which will manufacture and commercialise the PROSTACTO device. A second trial with patients monitored by La Fe is expected to be completed in 2025, so that the product can be brought to market in 2026 and implemented in La Fe itself and in private clinics in the Valencian Community. The intention is to enter the national and international markets in a second phase.
This development is a milestone for the IBV and REDIT, as RI Medical is the first start-up to emerge from the collaboration between REDIT and the technology centres of the Valencian Community, with the participation of La Fe, UPV and CSIC. It also demonstrates that the health technology sector in the Valencian Community can grow through synergies between different institutions.
Currently, in line with the European Cancer Action Plan, the collaboration is continuing with a view to intensifying screening for one of the most common cancers in the world, and improvements in design identified during the clinical research phase are being addressed. Our ultimate goal is to identify 18% of prostate cancers with normal PSA levels in an outpatient setting through rapid patient screening, thereby reducing mortality and improving the quality of life for patients diagnosed with this type of cancer.
BIBLIOGRAPHY and refereces
[1] Freddie Bray et al., «Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries», CA: A Cancer Journal for Clinicians 74, n.o 3 (2024): 229-63, https://doi.org/10.3322/caac.21834.
[2] «Las cifras del cáncer en España 2024», Informe anual (Sociedad española de Oncología Médica (SEOM), 2024), https://seom.org/prensa/el-cancer-en-cifras.
[3] «Las cifras del cáncer en España 2024».
[4] «Estrategia contra el cáncer de la Comunitat Valenciana 2019-2022» (Generalitat. Consellería de Sanitat Universal i salut Pública, 2019).
[5] «Estrategia contra el cáncer de la Comunitat Valenciana 2019-2022».
AUTHOR’S AFFILIATION
Instituto de Biomecánica de Valencia
Universitat Politècnica de València
Edificio 9C. Camino de Vera s/n
(46022) Valencia. Spain




