Human Centred Exoskeletons to enhance socio-health care market acceptance
27 December 2024.
Author (s): José Laparra Hernández, Juan Manuel Belda Lois, Laura Martínez Gómez, María Jesús Solera Navarro, Carlos Atienza Vicente.
Instituto de Biomecánica (IBV)
Exoskeletons can compensate or augment the capabilities of older persons and people with disabilities to support daily living activities, enhancing their quality of life and independent living expectative. In the process of design and development of exoesqueletons, a human-centred approach is necessary not only to overcome acceptance barriers of users, patients and clinicians, but also to reduce development costs and time to the market. Human Factors know-how (anthropometrics, biomechanics, thermal, mental, etc), User Experience and the most advanced human-centred laboratories are key to optimising exoskeleton-human interaction and speeding innovation process.
INTRODUCCIÓN
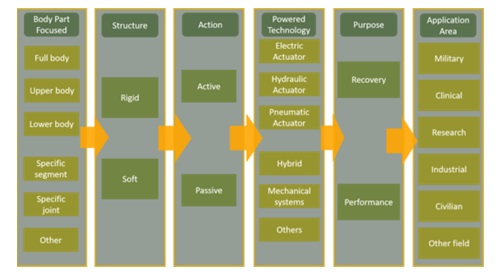
Exoskeletons or robotics wearables are devices externally attached to the human body to compensate or augment human capabilities (Figure 1). There are different classifications, being quite common to split at least among active or passive, and depending on its application area: military, clinical, workplace safety, etc (Figure 2). The concept is not new, in fact, one of first known device was patented in 1890 by Nicholas Yagin. However, the advancement and maturity in different domains (electromechanics, sensors, neuroscience, etc.) and the reduction of technology costs have impulse its deployment during the last decade.
Figure 1. Example of exoeskeletons to improve living conditions.
While the initial efforts were focused on solving technical challenges, with a clear technology push, nowadays there is a clear trend about the importance of performing a human-centred approach and involving users along the whole innovation process to enhance market acceptance, as it can be checked on the last specialized forums, trades and conferences[1]. This is essential for advancing these technologies from research and laboratory settings to real-world applications, where they can significantly improve the independence and quality of life for older adults and people with disabilities, as well as gain acceptance in the market.
Figure 2. Exoeskeletons classification (Tejera, 2020)
HOW TO ACHIEVE HUMAN CENTRED EXOESKELETONS?
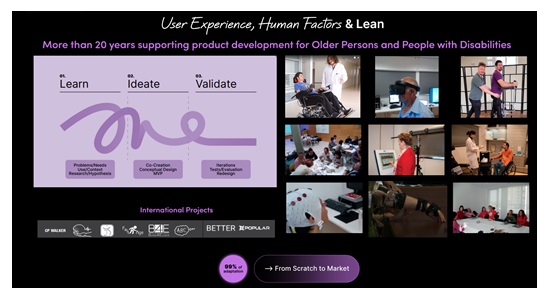
IBV has a proven track record of more than 20 years on supporting product development with a human-centred approach, covering products like, rehabilitation devices or wearables robotics. Our current approach is to combine Human Factors (HF), User experience (UX) and Lean to speed up the development process, reducing times, errors and costs (Figure 3), and to increase patients, clinicians and market acceptance. Our expertise can be grouped into 3 big areas:
- Know-how on human behaviour: anthropometry, biomechanics, postural simulation, thermal comfort, cognitive workload, emotional response, etc.
- Advanced Technology Labs to characterize humans and their interaction: mechanical tests, 4D shape, movement analysis, thermal response, physiological response, etc.
- Design and development skills, beyond electronics and mechanical engineering, covering accessibility, usability, interface design, etc.
Figure 3. Human-Centred Design approach to improve quality of life of older persons and people with disabilites
Depending on the stage of the innovation process, we use scalable technologies to assess human interaction in different contexts, from specialized laboratories, rehabilitation or physiotherapist clinics to the patient’s home. HF combined with UX methodologies allow us to support developers along the main phases: Learn, Ideate and Validate[1]. It should be highlighted the importance of detecting errors or design problems as soon as possible, because an error detected in a prototype phase has 10 times more impact on cost than during the conceptual design phase (Morales, 20024), and forces you to start your certification process again, delaying or hampering market arrival.
Moreover, IBV’s methodology complies with the current Medical Device Regulation (MDR), speeding up the certification process and simplifying the generation of the MDR documentation. In fact, IBV has medical device manufacturer license from AEMPS (Spanish Agency of Medicine and Medical Devices). Additionally, IBV has implemented ISO 13485 to ensure quality in our products and to support the design of software, electromedical equipment, and implant systems for third parties, supporting companies around Europe.
HF and UX go hand in hand to achieve excellent products and exoskeletons is not an exception. Although UX is key throughout the whole process, it is especially valuable during the Learning and Ideation Phases, allowing us to focus and work on the concept before wasting time and resources during the development phase.
While interviews, netnography, focus groups, etc. allow us to get in-depth and understand better the user needs, context and barriers (Learning phase), there are different methodologies to make the innovation process easier and more efficient along the Ideation phase. At IBV, we perform co-creation sessions with users, HF experts, designers and developers to generate new concepts with little effort leaning on methodologies like Braindrawing, Lego, etc (Figure 4). We also allow stakeholders to interact with other exoskeletons or technologies of other sectors to inspire them.

Figure 4. User Experience methodologies, from needs identification to cocreation.
On the other hand, HF goes beyond traditional physical ergonomics, covering physical, sensorial and mental domains, providing a holistic overview of human needs, capabilities and skills. In a nutshell, to apply our knowledge about humans to design products to optimize performance, safety, efficiency and satisfaction of exoskeleton-human interaction.
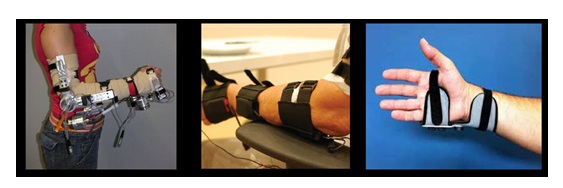
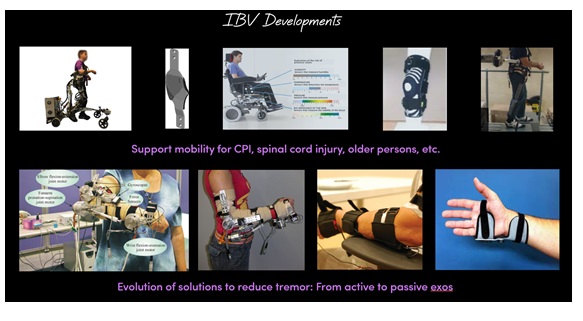
It’s better to focus on humans than only on technology because technology can rapidly change, especially over the last years. Figure 5 shows the evolution during the last decade from a bulky exoskeleton to a simple textile orthosis to minimize the tremor effect, which allows patients to comfortably perform daily life activities like drinking or opening a door with their keys, enhancing their independent living condition.
Figure 5. Tremor Reduction Evolution of IBV solutions, from exoeskeleton to textile wearables.
Next, there are some examples of how HF knowledge, methodologies and technologies can be applied to support the design, development and validation of exoskeletons.
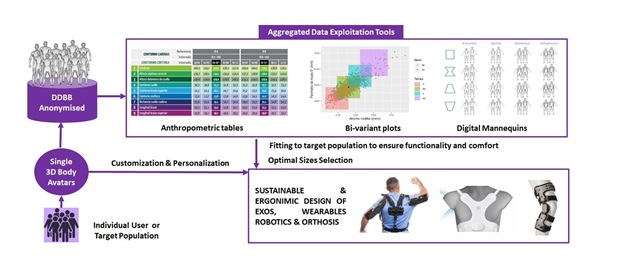
Anthropometry enhances adjustment to target population dimensions, allowing to solve, among others, the following issues (Figure 6):
- Balanced fitting to achieve enough pressure to ensure functionality but without affecting comfort.
- Optimal selection of the minimal number of sizes to cover the target population.
- Customization or personalization of the adjustments, supports, etc with very precise measures.
Figure 6. Anthopometric knowledge to improve design.
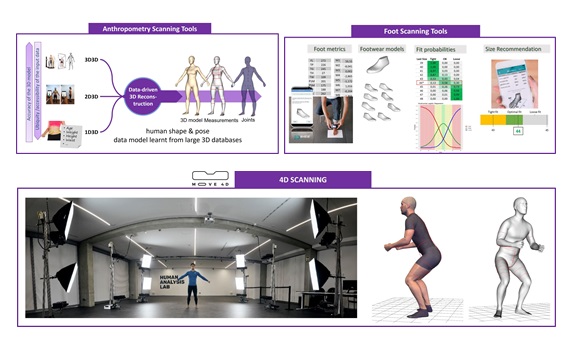
To achieve this, we have different scalable solutions, from 4D scanners with high resolution to accessible mobile applications such as 3D Avatar Body or 3D Avatar Feet (Figure 7). This is enhanced with our large realistic human database (>4.000 users from different countries) to identify the key anthropometric dimensions in order to cover 90% of the population or optimize the number of needed sizes.
Figure 7. Scalable Scanning tools for anthropometry.
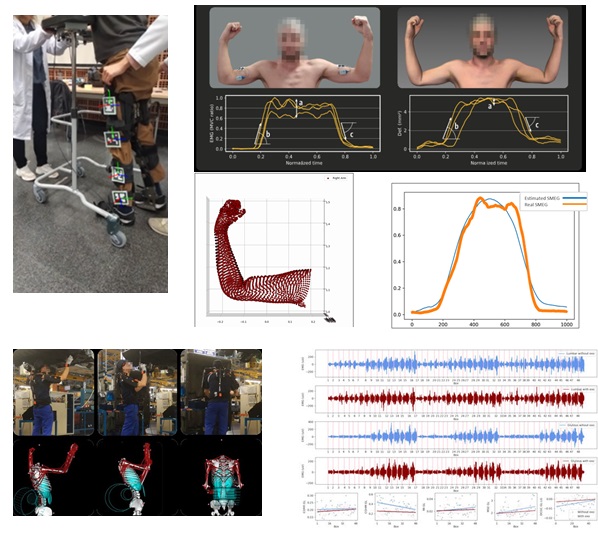
Biomechanics allows us to understand the body movement, muscles activation or muscular effort, among others. This is key to define and validate relative movements, allowing to develop control loops that follow the natural biomechanics of target population, for example, older persons. In this case, we also have scalable technology (markers, inertial sensors, normal cameras without markers or AR codes, EMG sensors, etc.) to move from the lab to any clinic environment or the patient’s own home (Figure 8).
Figure 8. Scalable technology to analyse movements and advanced analysis of physical effort and muscular activity.
Previous approaches can be combined with IBV know-how and technologies to measure forces, pressure or thermal response when using exoskeleton in order to achieve an optimal balance between performance and comfort, which is especially critical for the long-term use of these devices. And last but not least, especially when complexity increases or there is any biofeedback, we are able to assess interaction from a cognitive and emotional point of view, using different physiological measures such as EMG, GSR or HRV beyond traditional questionnaires.
HOW DOES IBV SUPPORT THE COMPANIES INNOVATION PROCESS?
IBV approach is not only about human-centred to optimize exoskeleton interaction but also to reduce time and costs of the design and development process, enhancing market acceptance during the last 20 years.
To sum up, we can support you leaning on:
- Know-How: Our expertise covers a wide range of areas including ergonomics, anthropometry, biomechanics, pressure, forces, movements (with and without markers), physiological signals (ECG, EMG, EEG, GSR), thermal comfort, cognitive workload, emotional response, etc.
- Human Factors Plan, User Experience & Lean: Providing support to accelerate the deployment of medical devices and enhance satisfaction among patients and professionals, based on user tests.
- Design and development of exoskeletons, including sensors integration, friendly interfaces (balancing usability and aesthetics), mechanics or electronics.
- MDR and ISO 13485 Compliance: We can support companies in complying with regulatory standards for their products, and provide certified designs by MDR of software, electromedical equipment, and implant systems for third parties.
Validation: IBV labs include facilities for anthropometry, movement analysis, thermal studies, extended reality, simulation interaction, user experience co-creation, and performance evaluation under real conditions. Moreover, we collaborate with different hospitals and associations allowing access to a large sample of patients and clinicians.

Figure 9. Users and Clinical Networks that collaborate with IBV.
ACKNOWLEDGEMENTS
We want to thank all companies that have trust in us.
Figure 10: Exoskeleton, support technologies and robotic companies that has worked with IBV to enhance independent living of older persons and people with disabilities.
BIBLIOGRAPHY AND REFERENCES
[1] https://www.exo-berlin.de/
https://2024.icneurorehab.org/
[2] https://www.ibv.org/campanya/human-factors/
De la Tejera, J. A., Bustamante-Bello, R., Ramirez-Mendoza, R. A., & Izquierdo-Reyes, J. (2020). Systematic review of exoskeletons towards a general categorization model proposal. Applied Sciences, 11(1), 76.
Morales Casas, A., López Vicente, Z., Solano García, L., and Laparra Hernández, J. (2024). Speed Up Medical Device Innovation: Combining Agile, User Experience, and Human Factors Methodologies. Production Management, Manufacturing, and Process Control (Eds. Beata Mrugalska, Waldemar Karwowski, Tareq Z. Ahram), Cap 6, Pag. 76-91. Edited By.
AUTHOR’S AFFILIATION
Instituto de Biomecánica de Valencia
Universitat Politècnica de València
Edificio 9C. Camino de Vera s/n
(46022) Valencia. Spain